Which Ions Have Noble Gas Electron Configurations
Chemistry 7th Edition Edit edition Solutions for Chapter 8 Problem 35E. Determine which ions have noble-gas configurations.
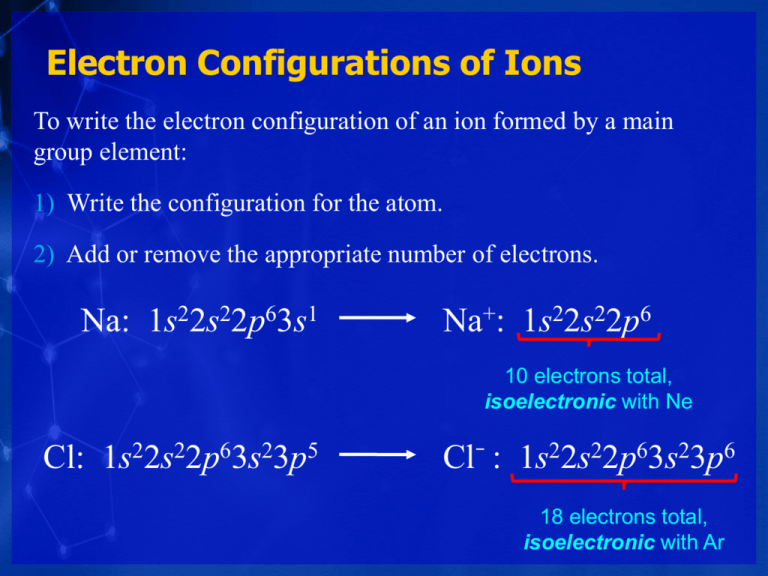
Electron Configurations Of Ions
The electron structure of a zinc ion Zn2 is an example of a pseudo noble gas formationThe lattice energy is the energy required to separate the ions of an atomic compound.
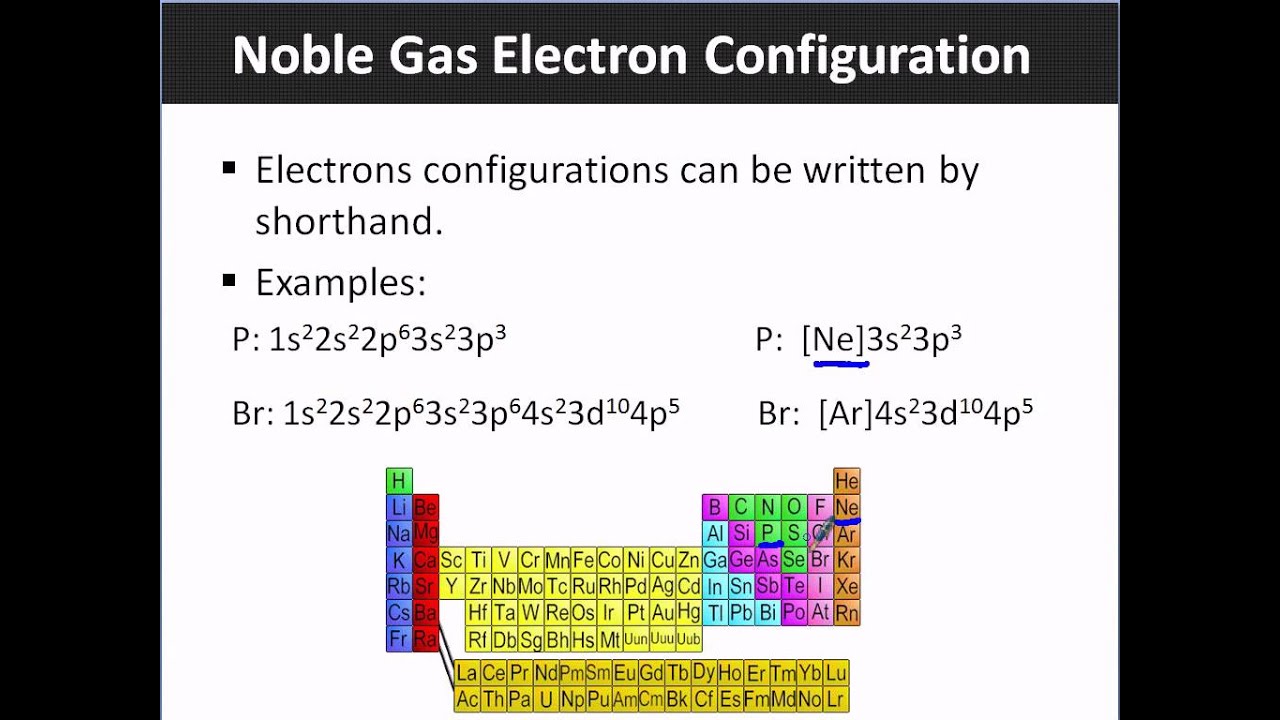
. So we move down and we see that we have our 4s electrons and we have two of them. I tried As3- and Zr4 but that was wrong so. Which of the following ions have noble gas electron configurations.
Carbon He2s 2 2p 2. So theyre losing electrons. Boron He2s 2 2p 1.
What is the electron configuration of a noble gas Why is this configuration important. Gain 3 e- to look like Argon P3-. The two additional electrons required to fill the valence orbitals give the oxide ion the charge of 2 O 2.
The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1s 2 2s 2 2p 6 3s 2 3p 6 the same as that of the noble-gas argon. Oxygen He2s 2 2p 4. So calcium the noble gas immediately preceding it we go up a row and then over and we see that the noble gas is argon so we write argon in our brackets and then what electrons are not in argon.
The resulting electron configuration for Oxide ion O 2- will be 1s 2 2s 2 2p 6. Finding the Closest Noble Gas Lithium 3 e-He 2 e-Ne 10 e-Helium is closer. Nitrogen He2s 2 2p 3.
HERE are many translated example sentences containing NOBLE GAS CONFIGURATION - english-indonesian translations and search engine for english translations. Lose 2 e- to look like Argon Ca2 Phosphorus 15 e-Ne 10 e-Ar 18 e-Argon is closer. It has to lose eight electrons total.
119 rows ELECTRON CONFIGURATION. Cd2 Ru3 P3 As3 Ag Zr4 Thanks for any help. Lose 1 e- to look like Helium Li Calcium 20 e-Ar 18 e-Kr 36 e-Argon is closer.
Barium is an alkaline earth metal group 2IIA so it forms Ba2 ions which have the noble gas configuration of xenon Xe. So Iron has thes air cat ions because they have positive charges. So Iron has two electrons in the outer circle.
Fluorine He2s 2 2p 5. Wiki User 2013. All alkaline earth metals have two valence electrons which they lose when forming ionic compounds.
Ions of groups 1 2 17 16 15 will generally have the electron configuration of noble gasesIons of transition metals may not have the electron configuration of noble gases. 2 10 18 36 54 and 86 respectively. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom followed by the configuration of the remaining electrons.
1 Introduction To Chemistry 2 Atoms Molecules And Ions 3 Equation The Mole And Chemical Formulas 4 Chemical Reactions In Solution 5 Thermochemistry 6 The. Atoms and atomic ions with sequences of completely filled electron shells exhibit enhanced stability. How to write the electron configuration for the Al 3 ion.
They are helium neon argon krypton xenon and radon. Which of the following ions have noble gas electron configurationsa. It resembles the configuration of the nearest inert gas ie Neon.
Click hereto get an answer to your question Which of the following ion do not have a noble gas electronic configuration. O 8 Na 11 Ca 20 Mn 25 l 53. Silicon Ne3s 2 3p.
Its got six in the D orbital and for to reach a noble gas configuration by Lucy Electrons that has to lose all of them. The sodium ions are sodium atoms which have lost an electron giving them the structure 1s 2 2s 2 2p 6 the same as that of the noble-gas neon. Noble Gas Electron Configuration eq1s2 2s2 2p6 3s2 3p64s2 3d5 eq eqAr 4s2 3d5 eq Chlorine Electron Configuration eq1s2 2s2 2p6 3s2 3p5 eq.
The prime examples are the noble gases He Ne Ar Kr Xe and Rn containing one of the magic numbers of electrons. Elements tend to react so that they acquire the electron structure of a noble gasA sodium atom tends to lose one electron when it reacts. Aluminum Ne3s 2 3p 1.
Neon He2s 2 2p 6. Select all that apply a V2 Ti2 Sc3 Fe3 b Se2 Cr2 Cd2 c Nb4 Ce4 Al3 d Ra2 Au Pd2 This problem has been solved. Therefore to write the electron configuration of O 2- ion we have to add two electrons to the configuration of Oxygen O.
Noble gas electron configurations are important because they have a total of eight valence electrons making an atom stable. The chloride ion now has the electron configuration of the noble gas argon 1s22s22p63s23p6. All electrons in both kinds of ions are paired.
Fe2 Fe3 Sc3 Co3b. These gases are colorless odorless and chemically inert although a few compounds of Kr Xe and Rn have. See the answer See the answer See the answer done loading.
Oxygen for example has the electron configuration 1s 2 2s 2 2p 4 whereas the oxygen anion has the electron configuration of the noble gas neon Ne 1s 2 2s 2 2p 6. So this is the noble gas configuration for calcium. Which of the following ions have noble gas electron.
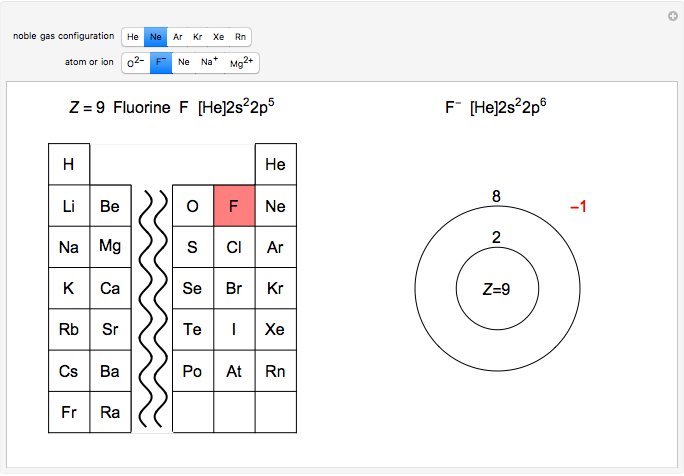
Ions With Noble Gas Configurations Wolfram Demonstrations Project
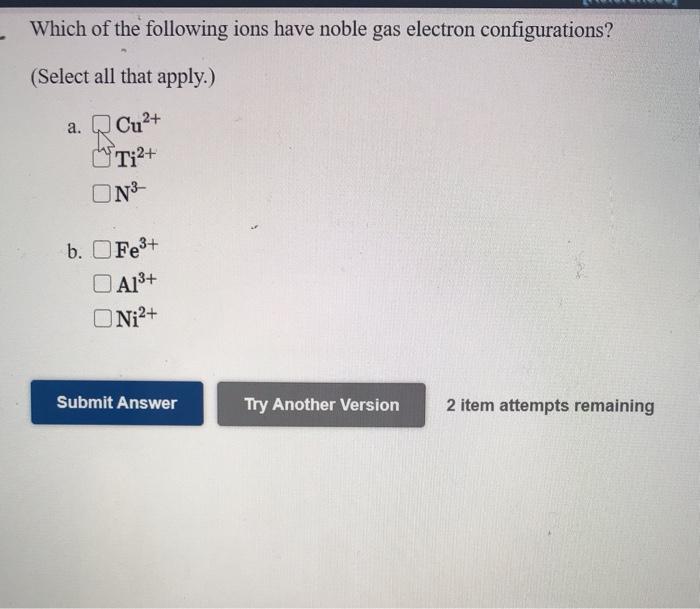
Solved Which Of The Following Ions Have Noble Gas Electron Chegg Com
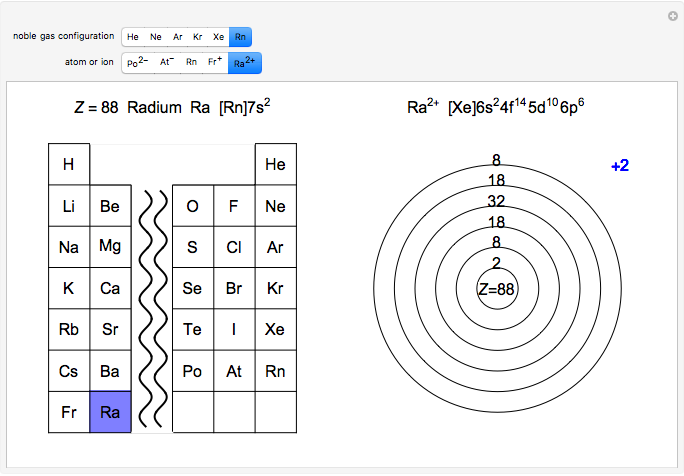
Ions With Noble Gas Configurations Wolfram Demonstrations Project

Comments
Post a Comment